The Partnership launches initiative to advance standardized cancer pathology reporting
July 18, 2012
 The Electronic Synoptic Pathology Reporting Initiative (ESPRI) aims to further the adoption and implementation of electronic synoptic pathology reporting in Canada to drive practice improvements across the cancer care continuum. Through this initiative, advancements will be made to the depth and consistency of pathology information collected leveraging the CAP cancer protocols (the national content standard for cancer pathology reporting), ensuring that the information necessary to provide timely, top-quality care is available to inform treatment decisions.
The Electronic Synoptic Pathology Reporting Initiative (ESPRI) aims to further the adoption and implementation of electronic synoptic pathology reporting in Canada to drive practice improvements across the cancer care continuum. Through this initiative, advancements will be made to the depth and consistency of pathology information collected leveraging the CAP cancer protocols (the national content standard for cancer pathology reporting), ensuring that the information necessary to provide timely, top-quality care is available to inform treatment decisions.
In close collaboration with provincial partners, these investments may include upgrades to infrastructure, support for systems integration to enable data transfer, as well as education and support to promote adoption of standards.
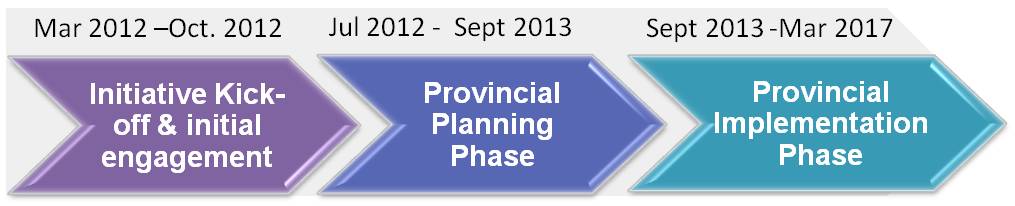
In order to achieve these targets and manage scope, a two-phased approach will be leveraged. Phase one will involve provincial planning, while phase two will involve provincial implementation(s) of electronic synoptic pathology solutions. The start of each phase will be preceded by the issuance of an RFP by the Partnership, to which interested parties can respond.
Current status: planning phase – SV2012-O1P Adjudication
For further details, please refer to the following documents:
- Electronic Synoptic Pathology Reporting Initiative RFP #1 (#SV2012-01P)
- Electronic Synoptic Pathology Reporting Initiative RFP #1 (#SV2012-01P) – Addendum #1
- ESPRI Introductory WebEx Details
- ESPRI Introductory WebEx Presentation
- ESPRI Introductory WebEx Recording
- Questions & Answers
- ESPRI Workplan and Budget Template Tool
- ESPRI Workplan and Budget Template Tool Guidance Document and Checklist
- Communication re: ESPRI Workplan and Budget Template Tool
At a high level, ESPRI will focus on:
- supporting the adoption and advancing the implementation of electronic synoptic pathology resection reporting in discrete field formats for breast, colorectal, lung, prostate and endometrial cancers
- maintaining and promoting the adoption of pan-Canadian mechanisms to support the necessary standards
- advancing the use of standardized data by generating performance indicators to measure completeness and compliance, as well as additional derivable clinical indicators
ESPRI provincial initiative in-scope:
- Planning and implementation of electronic synoptic pathology resection reporting in discrete field formats (level 5-6) at a recommended capture rate of 90% for all Breast, Colorectal, Lung, Prostate and Endometrial cancers utilizing the mandatory elements of the College of American Pathologists (CAP) Cancer protocols
- Enabling the electronic generation of indicators by provincial partners to measure:
- compliance to the CAP Cancer protocols (% of reports and institutions leveraging discrete field formats)
- completeness (% of mandatory fields complete)
- potentially derivable clinical indicators
- Integration of electronic synoptic pathology data into existing e-Health infrastructure, including:
- provincial Cancer Registry
- provincial Lab System (where applicable)
- provincial EHR (where applicable)
- institutional HIS
Provincial initiative out-of-scope:
The Partnership will not fund the following components which either do not support the goals of the Initiative or have been funded through other initiatives:
- implementation of systems which do not support the CAP eCC XML or equivalent
- implementation of systems which do not support CHIs electronic synoptic pathology Canadian Approved Standards
- voice recognition/dictation based systems
- e-Path related products
- digital and tele-pathology image scanning
- vendor certification (i.e. working directly with vendors to provide certification offerings)
As stated, in order to achieve these targets and manage scope, a two-phased approach will be leveraged. Phase one will involve provincial planning, while phase two will involve provincial implementation(s) of electronic synoptic pathology solutions. Timelines for the Initiative and the ESPRI RFP #1 are as follows:
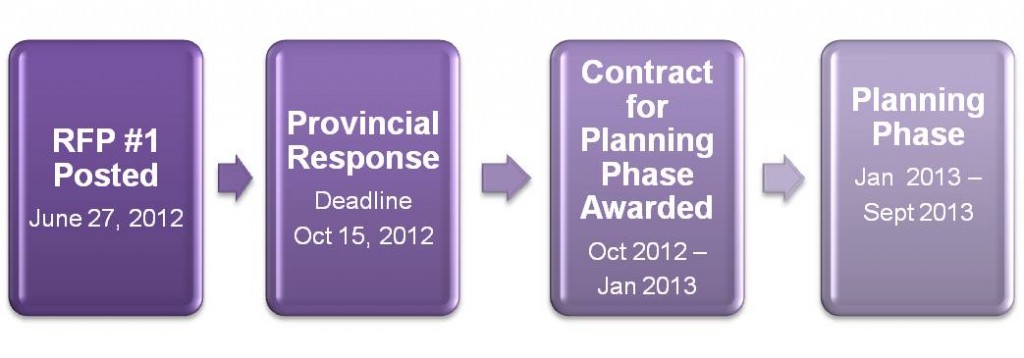
The ESPRI RFP#1 timeline for posting, response and subsequent planning phase is as follows:
For more information please contact: pathology@partnershipagainstcancer.ca
